My Ph.D Research
My Ph.D work aims to explore the role of actin regulatory proteins in the molecular mechanics of smooth muscle efficient force maintenance.
There are three types of muscles in your body: the muscle that you build in the gym (skeletal muscle), the muscle that beats your heart (cardiact muscle), and the muscle that moves your intestines, controls your blood pressure, and causes uterine contractions during delivery (smooth muscle).
Smooth muscle disregulation can causes many different diseases. Some examples are hypertension, asthma, gastrointestinal disorders, and urinary incontinance. Despite this fact, very little is known about how this type of muscle contract and, most importantly, how it stays contracted.
Some types of smooth muscle have this remarkable ability to maintain force with very low energy consumption, a phenomenom that us scientists like to call the “latch state”. Very little is known about the latch-state. That is because studying the molecular regulation of smooth muscle has historically been challenging due to the lack of advanced technologies capable of probing molecular mechanics within a dynamic environment.
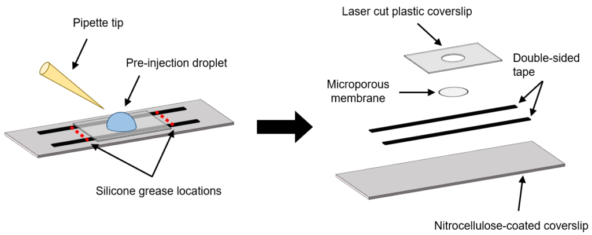
My home laboratory (Dr. Anne-Marie Lauzon’s lab) recently developed specialized diffusion chambers that enable precise manipulation of assay conditions, including the introduction of enzymes, without creating any bulk flow that would disrupt molecular mechanics measurements1. Coupled with the 2018 physics Nobel Prize winning laser trap assay, these novel chambers can be used to measure forces produced by molecular motors in a dynamic environment.
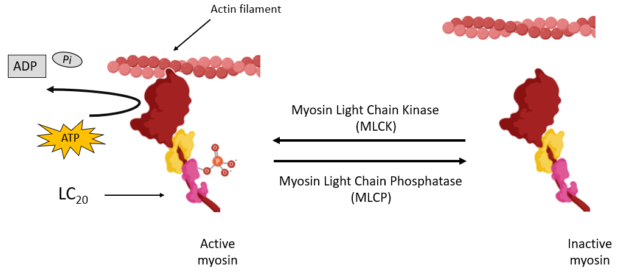
Smooth muscle contraction is produced by the cyclical interaction between myosin, a molecular motor, and actin filaments. This interaction is regulated via the activation of myosin through the phosphorylation of its regulatory light chain (LC20) by myosin light chain kinase and deactivation by myosin light chain phosphatase (MLCP). The classical model of SM force maintenance stipulates that it occurs when myosin gets deactivated while attached to actin. However, this model fails to incorporate other regulatory elements of SM contraction such as actin regulator proteins (ARPs). ARPs, namely h-caldesmon (hCaD), calponin (CaP1) and tropomyosin (Tm), are believed to contribute to SM force maintenance. However, the precise molecular mechanisms remain unclear.
Therefore, my Ph.D work aims to harness innovative instrumentation for molecular mechanics measurements with my personal training in biochemistry and bioentineering to explore the role of ARPs in the molecular mechanics of smooth muscle efficient force maintenance.
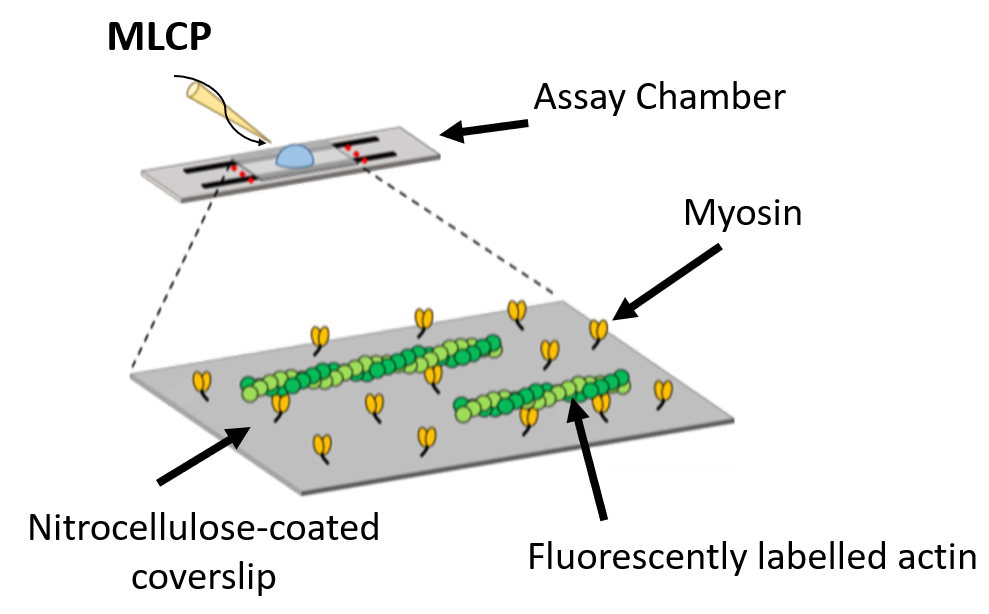
The In-Vitro Motility Assay
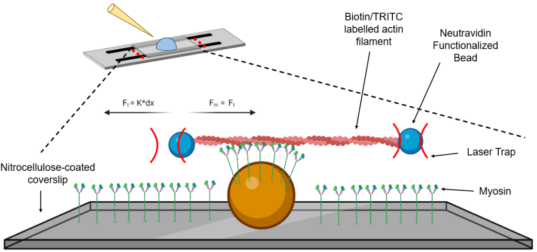
The Laser Trap Assay
Past Projects
A Method for Isolating Contractils Smooth Muscle Cells from Cryopreserved Tissue
When I first joined the Dr. Lauzon’s lab, I was working on the development of a protocol for the isolation of contractil smooth musce cells (SMCs). Many techniques had been developed to isolate contractile SMCs from tissue with varying degrees of success. However, most of these approahces relied on obtaining fresh tissue, which posed logistical challenges. We introduced a novel protocol for isolating contractil SMCs from cryopreserved smooth muscle tissue, in an attempt to enhance experimental efficiency.
Check out the published article!
Cellular Lipid Droplet Induced Cell Growth Inhibition is Off-Set by Overexpression of TPD52.
Non-alcoholic fatty liver disease (NAFLD) is the chronic hepatic expression of metabolic syndrome that affects close to 20% of Canadians. It is characterized by the accumulation of cellular lipid droplets (CLDs) in hepatocytes, and it is often accompanied by other metabolic disorders such as obesity and type two diabetes. A hallmark of this chronic disease is the transition of a non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH) which consists on inflammation of the liver accompanied or not by fibrosis that can then lead to cirrhosis. Understanding of lipid metabolism is vital to understand the development of this disease, especially when it comes to lipophagy controlled by mTORC1. Proteins bound to CLDs are promising factors in the regulation of lipid metabolism. TPD52 was found to localize to lipid droplets and to interact with LAMTOR1, suggesting that TPD52 plays a role in lipophagy through regulation of mTORC1. In this study, the growth of HEK293-TRex cells expressing a tetracycline-inducible TPD52 gene after addition of oleate was monitored. It was observed that increased levels of oleic acid to induce CLD formation inhibits cellular proliferation and, that increased expression of TPD52 effectively overcomes such inhibition. We hypothesize that CLD-induced growth inhibition (as in NAFLD) is off-set by the increased expression of TPD52 to maintain cellular regeneration. Also, such a scenario increases the risk of HCC.
This was an undergraduate research project that I did under the supervision of Dr. Robert Kiss.